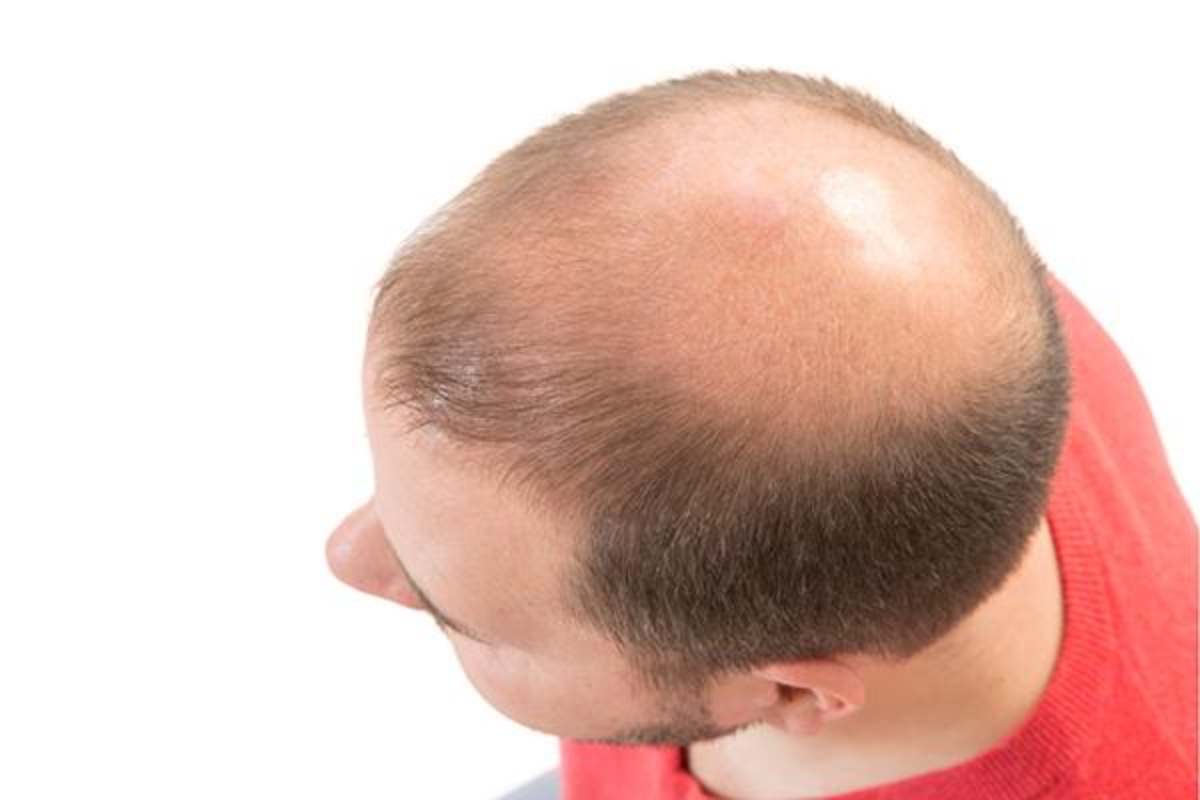
Hair Loss Benefits of Finasteride
Approximately 85% of individuals who routinely use finasteride see a stabilization of their hair loss or dramatic slowing of the loss.
Over 65% of patients who use this medication see an actual increase in hair numbers. While we typically see a greater response on the crown, it also helps the mid-portion of the scalp and, to a lesser degree, the frontal region.
The vast majority of patients will see an increase in hair weight. This means more volume of hair even if the actual numbers do not increase.
Side effects are rare, and there are no reported medication interactions between finasteride and other prescription medications. You should always consult with your primary physician before starting a new medication.
About Finasteride and Dutasteride
This category of drugs is known as 5-alpha reductase inhibitors. These medications prevent testosterone from being converted to DHT (dihydrotestosterone) in the prostate, hair follicles, and oil glands. DHT is the active form of testosterone that causes hair loss. The two prescription medications available in this class are finasteride (Propecia and Proscar) and dutasteride (Avodart). Neither drug blocks testosterone activity throughout your body as a whole, only in the specific areas that contain these enzymes. Finasteride 5mg & dutasteride are FDA-approved to treat benign prostatic hyperplasia (BPH or enlarged prostate). Only finasteride 1mg is FDA-approved to treat hair loss. Dutasteride can be used to treat hair loss, but this is considered off-label.
The vast majority of men (and some women) can benefit from these medications without adverse effects.
- Women who are pregnant or potentially could become pregnant CANNOT take these medications, as they interfere with the developing baby’s hormones.
- You CANNOT donate blood while taking these medications because a pregnant woman might be the one who receives your blood.
- Tell your doctor you are taking one of these medications as they can lower your PSA level. PSA tests are used to screen for possible prostate cancer development.
- Although there is no proven risk to the fetus, men may choose to stop this medication if they and their partner plan to conceive a child.
Fun Fact: Saw palmetto, which has gained popularity as both a stand-alone supplement and a component of many over-the-counter hair vitamins, is thought to improve hair growth by inhibiting 5-alpha reductase. Though less potent than finasteride and dutasteride, saw palmetto could be a good place for the medication-weary patient to begin.
Finasteride and Dutasteride Side Effects
Decreased sex drive and difficulty in achieving an erection has been reported in ~2% of men using these medications compared to placebo groups. In all major studies, the side effects went away upon discontinuing the use of the medication. There have been rare reports of men who claim to continue to have problems after stopping the medication. In most of these reports, the men continued taking the medication for several years despite of their symptoms. A class action lawsuit is ongoing claiming “Post-Finasteride Syndrome.” The true validity of this syndrome is still under debate and research. More recent data suggest that men with concomitant hypertension, diabetes, obesity, tobacco use, and mood disorders / anxiety, all of which could cause sexual dysfunction themselves, may be at higher risk of reporting decreased libido or erectile dysfunction on a 5-alpha reductase inhibitor.
Breast or testicular tenderness can be seen but is rare (<1%) and goes away upon stopping the medication.
Allergic reactions are possible but has not been seen in any of our patients at Limmer Hair Transplant Center, despite prescribing these medications thousands of times over the years.
Depression – While not reported as a side effect in any of the major studies, there have been rare reports of depression in the literature and on the internet. The validity of these reports remains unclear.
Decreased sperm counts – While not a reported problem during FDA trials, there have been rare cases reported in the literature of and a positive link between use and decreased sperm count. In trials evaluating this side effect, upon discontinuation, sperm counts returned to normal within 3 months.
Breast cancer is very rare in men in general and no association between using these medications and breast cancer has been shown; however, if you experience any lumps, bumps, pain, or nipple discharge, you should report it to your physician. Women who have a history of hormone-positive breast cancer will need permission from their oncologist to take these medications. That being said, many oncologists do give dermatologists the go-ahead to prescribe finasteride or dutasteride to their patients, especially after 5-10 years of surveillance without recurrence.
Prostate cancer is the 2nd most common form of cancer in men in the United States, and over 15% of men will be diagnosed with it during their lifetime. Prostate cancers are graded on a Gleason Score scale from 1 to 10. The vast majority of prostate cancers are low to mid grade types with Gleason Scores of 6 or less. In two large clinical trials evaluating these medications, there was a 15-25% reduction in the incidence of prostate cancer. However, if you developed prostate cancer during the study period, there was a small increased risk that your cancer would be a higher grade Gleason Score (8-10). For those taking 5mg of finasteride, the risk was 1.8% vs. 1.1% on placebo, and for those taking dutasteride, the risk was 1.5% vs. 1.0% on placebo. The data regarding the link between these medications and possible increased/decreased risk of prostate cancer remains controversial and under intense review.